Der erste Patient in Deutschland wurde im Rahmen der Immatics‘ ACTengine® IMA202-101 Studie behandelt Die deutsche Zulassungsbehörde, das Paul-Ehrlich-Institut (PEI), hat den Antrag (CTA, Clinical Trial Application) für eine weitere […]
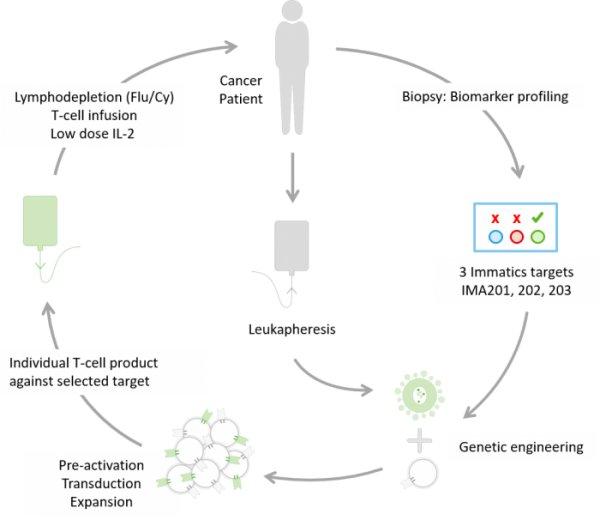

Der erste Patient in Deutschland wurde im Rahmen der Immatics‘ ACTengine® IMA202-101 Studie behandelt Die deutsche Zulassungsbehörde, das Paul-Ehrlich-Institut (PEI), hat den Antrag (CTA, Clinical Trial Application) für eine weitere […]
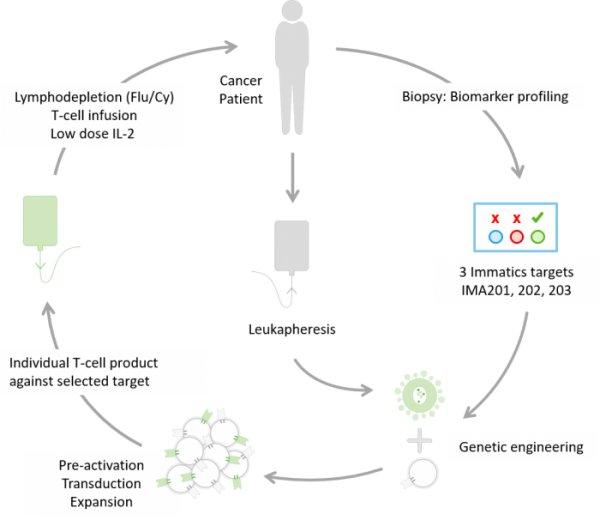
First patient has been treated in Germany in Immatics’ ACTengine® IMA202-101 trial German regulatory agency, Paul-Ehrlich-Institute (PEI), granted approval to commence another clinical ACTengine® trial in Germany investigating Immatics’ IMA203 […]
. Extension of collaboration between UTHealth and Immatics until end of 2024 providing Immatics exclusive access to state-of-the art cGMP manufacturing infrastructure at The Evelyn H. Griffin Stem Cell Therapeutics […]
Immatics N.V. (NASDAQ: IMTX, “Immatics”), a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, today announced changes to its Board of Directors in conjunction […]
Arya Sciences Acquisition Corp. (NASDAQ: ARYA or “Arya”), a special purpose acquisition company sponsored by Perceptive Advisors, and Immatics Biotechnologies GmbH (“Immatics”), a clinical-stage biopharmaceutical company active in the discovery […]
Immatics Biotechnologies GmbH, a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting cancer immunotherapies, today announced that Cedrik Britten, MD, has been appointed as Chief […]